The Human Holobiont
Realizing Contagion
Modern ideas about contagion and disease have their origins with the ancient Greeks. Our best evidence of what ancient understandings look like comes from a collection of works known as the Hippocratic Corpus, a collection of 60 ancient works associated with Hippocrates and his teachings. These works are credited with developing the first natural understanding of disease. Instead of leaning on supernatural explanations for things like epilepsy (previously considered punishment from the gods), the Corpus suggests that this is a disorder of the body, and can be treated as such.
The same is clear for the Hellenic perspectives on contagious diseases. Despite not having a clear understanding of the microscopic world, the Corpus suggests that disease is caused by the patient being exposed to “miasma,” or bad air. This is taken from a passage in Nature of Man, which first points out that diseases come for people no matter what they eat, how they sleep, or exercise. Given that the cause “is not regimen; it is the air that we breathe which is the cause; and it is clear that the air is harmful because it contains a pathogenic emanation.”
What this suggests, is that as early as 400BC, roughly 2400 years ago, there existed a primordial understanding that there could be something invisible that caused disease that spread through the air. However, there were two major obstacles to accepting this perspective. The first was a mystical belief that something could come from nothing, and the second was the fact that the transmission vector was invisible.
Animalcules from a letter by Leeuwenhoek to the Royal Society on the animalcules in the roots of Duckweed, December 1702
It was the second obstacle – that we couldn’t see the objects responsible - that was overcome first. The optical microscope was invented in the mid 1600s, allowing natural philosophers (as they were called then) to pursue the very, very small. Robert Hooke’s illustrated manuscript Micrographia was so popular that many, to this day, believe he invented the microscope in the first place. But Hooke and those who came before him were limited to microscopic observations of larger structures – the compound eye of the fly, the empty spaces in a piece of dessicated cork. The first person to observe microscopic organisms was Antoine van Leeuwenhoek, a draper from Delft whose findings were never presented anywhere except for in letters to the Royal Society, filled with drawings of his “animalcules.”
However, little attention was paid to the vibrant world under our noses for another 150 years. The causes are complex. When Hooke, Leeuwenhoek, and Malpighi died, they had no successors, and microscopy simply fell out of fashion. Given there was no clear path to making a living off of it, few were interested in following in their footsteps. Why was there no money to be made in microscopy, you might ask – and I would suggest that the doctrine of spontaneous generation, which was not very well settled by this point, had something to do with it.
Spontaneous generation is the belief that something can come from nothing. Today we realize that this would violate fundamental principles of thermodynamics – but humans didn’t always feel this way. Many historical understanding of how the world and the objects in it were formed,were based around the teachings of the Church. Natural philosophers interested in exploring the origins of objects were effectively hamstrung by the fact that right there in the first line of the King James Bible was that something could come from nothing - In the beginning God created the heaven and the earth.
Given that Giordano Bruno, the first to propose that stars had their own planetary systems was burned at the stake for heresy, and that Galileo Galilei, vocal advocate of heliocentrism, was kept under house arrest for the ten years preceding his death, coming up against the Church in matters of the natural world was not a wise decision. It actually took almost 200 years of active debate after the first microorganisms were observed to settle the question – can life come from inanimate objects?
In the late 1880s Pasteur and Tyndall developed a sufficiently convincing demonstration that laid the question to rest. They took a swan-neck flask of broth, and sterilized the contents. The swan neck allowed the flow of air into the flask, but its shape prevented the passage of any microorganisms. For as long as the swan neck was intact, the broth would remain sterile. When the neck was broken off, and there was no longer a physical obstacle between the liquid and the open air, the broth quickly became contaminated. This simple experiment, carefully carried out, demonstrated contagion of sterile places was something carried in the air – rather than a vital force in the air itself. It was a transformation from a spiritual mechanism to a physical one, akin to the one proposed in the Hippocratic Corpus more than two thousand years before.
Contagion, Disease, and the Humble Microbe
The demonstration, underpinned by the work of Pasteur and Tyndall, allowed for a physical study of disease. No longer bound by the supernatural explanation of “bad air,” and disease as a punishment for evil actions, scientists could turn to teasing out the actual cause and effect.
Based off of his understanding of microbial processes, Pasteur invented a process that would prevent the diseases of wine and beer. He suggested that these diseases were caused by an overgrowth of bacteria, which turned the alcohol to vinegar. By his reasoning, if brewers heated their mash to temperatures that were hot enough to kill bacteria but not hot enough to kill yeast, that their brews would be protected. And he was only about 300 years behind scientists in China and Japan with this realization! But those scientists failed to turn their knowledge into an applicable industrial process, so it’s called Pasteurization rather than Tamonin-nikkization today.
Pasteur’s demonstration, that undesirable microorganisms could cause diseases of beer and wine, was extrapolated to human health and soon began to pay dividends. Within a few decades, causative agents were found for diseases like diphtheria (Corynebacterium diphtheria), typhoid (Salmonella ser. typhi), cholera (Vibrio cholerae), yellow fever (Flavivirus), tuberculosis (Mycobacterium tuberculosis), and the bubonic plague (Yersinia pestis). The link between these agents and the diseases they cause was tested through empirical measurements by scientists like Robert Koch, Friedrich Loeffler, and Friedrich Henle – physicians that empirically demonstrated that there was such a thing as an infectious agent, an actual physical object that could cause a diseased state.
Their findings were memorialized in something called the Koch-Henle postulates, a list of requirements that must be met in order to connect a certain microorganism to a disease:
The microorganism must be found in abundance in all organisms suffering from the disease, but should not be found in healthy organisms.
The microorganism must be isolated from a diseased organism and grown in pure culture.
The cultured microorganism should cause disease when introduced into a healthy organism.
The microorganism must be re-isolated from the inoculated, diseased experimental host and identified as being identical to the original specific causative agent.
The interesting thing about this list is that it was almost immediately apparent it was incomplete; it failed to take into account the complicated landscape of host-pathogen interactions. Asymptomatic carriers, individuals that could infect others while experiencing no symptoms of the disease invalidated the first count. Difficulties in growing the pathogen in question in pure culture, especially for viruses, invalidated the second. Immunity, either through genetic changes, vaccination, or previous exposure invalidates the third point. Horizontal gene transfer and viral recombination has shown that the fourth postulate is shaky as well.
Despite all these flaws, the postulates served as a starting point for the next century of medical intervention against disease. The early to mid 1900s was the golden age of the pharmaceutical industry. They were able to produce antibiotics that just worked, and vaccines that brought diseases of flesh like smallpox under control. However, the second half of the 20th century led to a significant shift in our understanding of disease, vectors, and the fundamental principles of germ theory.
The Silver Bullet
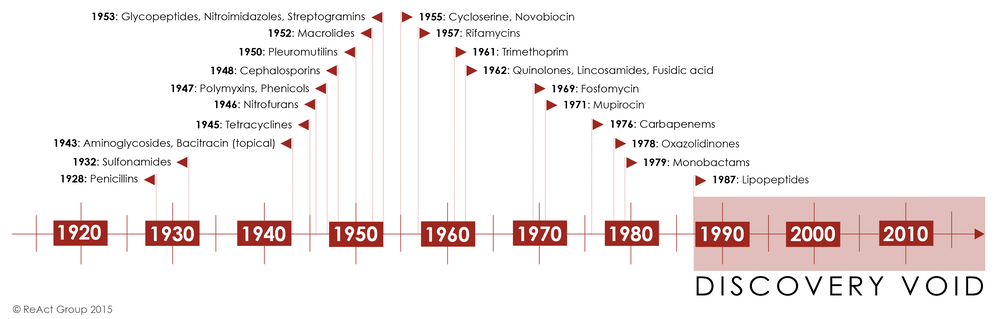
Discovery of penicillin by Alexander Fleming in 1928, and the subsequent purification and pharmaceutical application of it was an enormous breakthrough. It created a world where a life-threatening infection could be reversed. It suddenly seemed that, in a world filled with infectious agents ready to cause disease, humans had found the silver bullet.
However, this was a short-lived victory. Bacterial immunity against highly purified chemical treatments like penicillin began to appear as early as the 1940s. By the late 1960s, 80% of all Staphylococcus aureus infections were resistant to the antibiotic. There were other treatments, of course, but the trend was similar. Widespread use led to decreased efficacy, measured as a rise in resistance. The drawer of antibiotics, filled to the brim through the 1970s, suddenly looked barren.
The golden age of antibiotics discovery, driven by pharmaceutical companies that found a sweet spot between the ease of discovery and toxicity of application. In the later part of the century, discovery slowed significantly, while at the same time resistance to established treatments began to grow.
The problem with all these drugs, and the reason that there has been a rise in resistance in almost every pathogen that these drugs have been used to fight, is that there were errors in the way we understood antibiotics. The paradigm of germ theory and Koch’s postulates was that there was a war between humans and pathogens, and that antibiotics were magical weapons that would allow us, once and for all, to triumph over infection, sickness, and disease.
Bacteria, however, have a different relationship to antibiotics. Recent work suggests that they’ve been evolving mechanisms for dealing with antibiotics for thousands of years. Excavated permafrost sediments from around 30,000 years ago showed that the bacteria contained within already possessed the genomic information necessary to confer resistance to vancomycin, a glycopeptide antibiotic (isolated from Borneo soil samples in 1953). Further research has demonstrated that administration of low concentrations of all antibiotics, regardless of family or mechanism of action, leads to changes in gene expression, rather than overt toxicity. In many case, these changes in gene expression actually accelerate bacterial growth, rather than prevent it.
What these findings suggest, especially when viewed through the lens of the Koch-Henle postulates, is that the last century has created an overly simplistic scientific understanding of how microorganisms function in the complex ecological system that is the human body. Sequencing the human genome, and the subsequent fall in the cost of sequencing, has pushed the limit on how we understand microbes, infection, and disease – but the more we discover, the less clear the picture becomes.
In Sickness and In Health
The effect of the microbiome on human health has been a major area of study for the last 17 years – and has resulted in the realization that the presence of the pathogen does not, in and of itself, result in disease. Almost a third of the population normally carries Staphylococcus aureus, the bacterium responsible for serious skin infections. Typhoid Mary was outwardly healthy, but carried a lifelong Salmonella typhi infection, infecting more than 50 people over the course of her life. Latent tuberculosis infections are so widespread, that the asymptomatic phase is actually part of the disease description.
The complexity of health and disease, infection and contagion, also expands into the world of viruses. Being able to study the virome is a recent development, strongly influenced by the new sequencing approaches that allow for enrichment of viral DNA vs bacterial or host DNA. Sequencing of all viruses carried by humans has led to the realization that here, too, there is a less-than-clear correlation between presence of a given virus and the disease that it causes. HIV, viral infection that has killed millions worldwide, can lie dormant in the body, apparently controlled by a highly functional immune system.
It’s been difficult to find this kind of information, largely because not that many people have been searching for it. It’s rare to test a patient for an HIV infection when they appear perfectly healthy, and those that have fallen sick are unlikely to be “asymptomatic carriers,” by definition. What this means, is that the full diversity of the viral community that lives inside of humans is still hidden, despite how cheap sequencing has become.
One obstacle on the path to understanding the relationship between viruses and their hosts, is the huge amount of sequencing that must be done in order to determine the viruses that are least abundant in a sample – which, by definition, would be the asymptomatic pathogenic viruses in a carrier that did not show any symptoms of the disease. From a paper on sequencing the marine virome in San Diego:
The most abundant virus in both marine communities comprised 2–3% of the total population. If this genome is ≈50 kb, only 25,000 clones would have to be sequenced to get 10× coverage of this virus. Similarly, ≈500,000 clones would have to be sequenced to get 10× coverage of the 100 most abundant viruses. The Joint Genome Institute is currently sequencing ≈206 96-well plates per day (May 1, 2002, at www.jgi.doe.gov/). This is a total of ≈19,776 clones per day. Therefore, the sequence of the most abundant virus could be obtained with 1 day's worth of sequencing effort, and the 100 most abundant viruses could be sequenced in 1 month. The current library contains ≈1 million clones, with enough viral DNA remaining to create 15 more libraries of this size. Assuming our diversity estimates are correct, there is enough DNA in our sample to sequence the genome of every virus in the marine communities, even though this would require extraordinarily large sequencing efforts. These results show that it is not only possible to sequence the entire genome of an uncultured marine virus by using this approach, but also to sequence the entire genome of an uncultured marine viral community.
Advancements in enrichment techniques have accelerated the exploration of the human virome in recent years. But for the most part, viruses have remained hard to study. Though they outnumber cells anywhere from 10:1 to 871, they contain far less genomic information – which means that their nucleic acids are far less abundant in a given environment. Low abundance of nucleic acid can make genome assembly difficult, which means that virions must be purified away from any other cells present in the sample that contain contaminating genetic information. Many selection schemes depend on size selection, so any large, cell-sized viruses are filtered out before purification is complete.
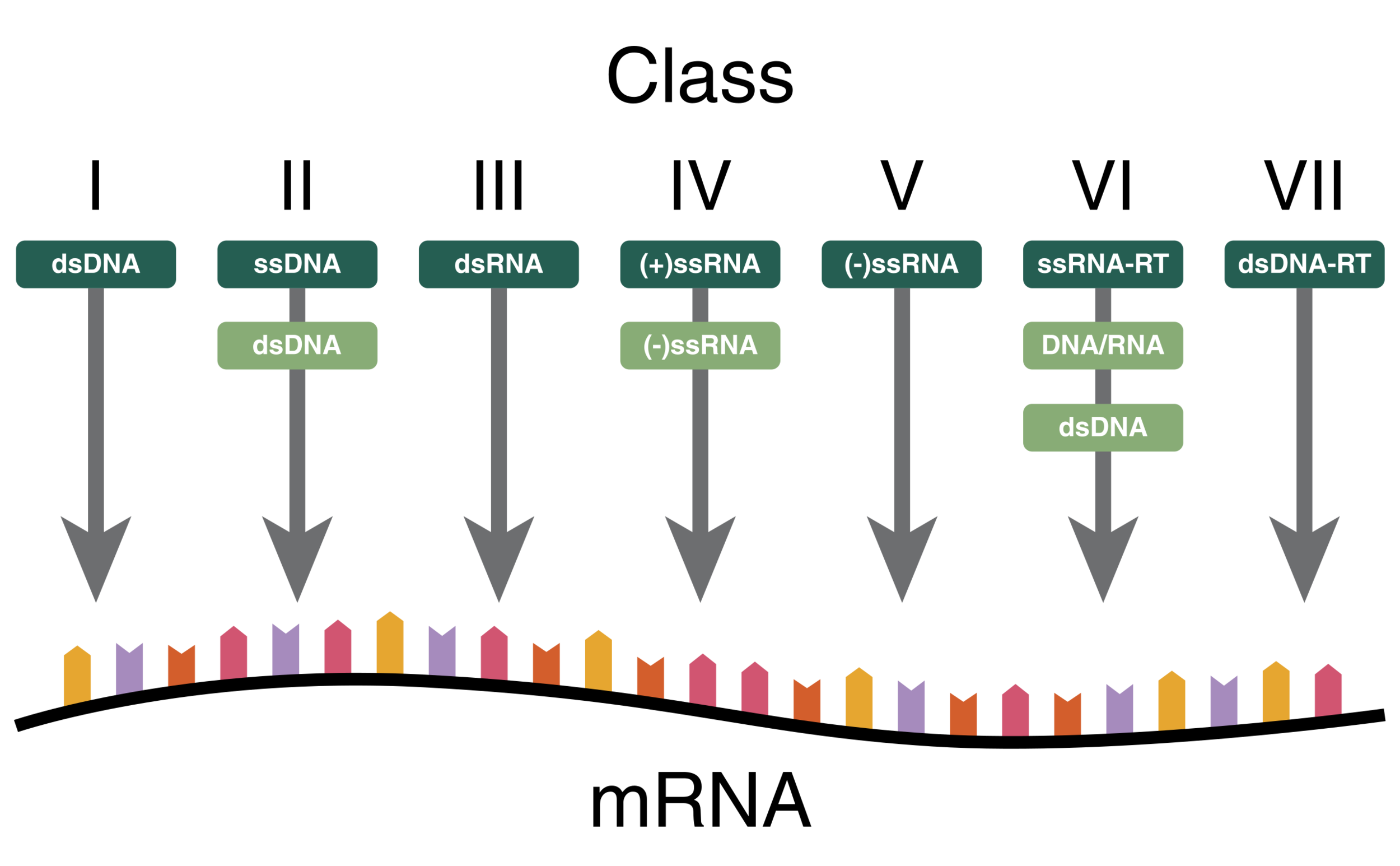
Different nucleotide schemes used by viruses. In dark green is the starting genetic material, in light green are any intermediate steps necessary to produce mRNA, the substrate used by the ribosome to produce viral proteins.
Purified virions are almost ready for sequencing and genome assembly, but there’s one more confounding step – nucleic acid amplification. All viruses, no matter their nucleic acid content, will eventually produce mRNA once they’re in the cell. However, the starting point can be one of seven different configurations – but amplification programs are usually limited to targeting one type of nucleic acid at a time, resulting in an additional filtering step before genome assembly can be carried out.
Despite these difficulties, researchers are making advances into studying viruses – at this point, mostly the abundant ones, but the need for more sensitive detection methods is widely accepted in the literature.
The Human Virome
Broadly speaking, there are two groups of viruses found in human virome studies – phages, those that infect bacterial and archeal cells, and eukaryotic viruses - those that infect cells with a nucleus and organelles. Given the enormous amount of bacteria in the human body – something like a pound of bacteria per person – it’s no surprise that phages are a huge part of the human virome. By some estimates, phages in the gut outnumber bacteria by an order of almost 100:1, an even higher ratio than the 10:1 seen in seawater. The number of virions is highest in barrier areas, like the skin and the intestines (109) – but is still significant in the blood and the urine (105).
The question of where viruses come from in the body can be interrogated by studying changes in newborns. Shortly after birth, “Direct epifluorescent microscope counts did not detect any viral particles in the meconium (i.e., the earliest infant stool samples). By the end of the first week, however, there were 108 virus particles per gram wet weight of feces.” That is to say, that infants are born without detectable virus levels in their guts, and quickly become populated with new phages. The same study that looked at the number of viruses in meconium pointed out that the majority of metagenomic sequences of the infant fecal microbiome don’t correspond to any known viruses – suggesting an almost entirely novel population of viruses that appears in a newborn.
These viruses could come from the environment – or they might come from inside the bacteria themselves, as one team discovered that the majority of viral genetic material identified in a metagenomic study belonged to prophages – bacterial viruses that are incorporated into the genomic DNA of their bacterial hosts.
In some cases, the variation between human metagenomes is quite high – in a fecal metagenomics study done on three Singaporean individuals, there were 42 viral genomes assembled – and only two of them were present in all three individuals. Another study, looking at the stability of the virome in the stool of a single individual over the course of almost three years, found that the spread of viruses present in the intestine stayed constant over a 2.5 year period. This doesn’t necessarily say that it will always stay constant, but it does suggest that there are components of individual viromes that are unique to an individual and, more than that, persist over time.
Other studies show that there are viruses that appear to be present in the majority of people tested. The hilariously named crAssphage was recently mined from a fecal metagenomics study of a mother and twins – appears to be widely distributed across the world. Almost 1/3 of the samples of human viral metagenomes contain the crAssphage. Despite its abundance, it hadn’t been previously identified due to the difficulty of assembling multiple novel virus genomes from a highly variable sample. Authors of the study that identified the crAssphage suggest that there is an enormous abundance of other common phages waiting to be discovered… but they declined to discover them in this same paper.
In terms of direct effects of phages on human health, the evidence is unclear. Phages certainly affect the composition of the microbiome, providing a top-down control of bacterial populations through what is called kill-the-winner dynamics. Any bacterial species that outcompetes others is vulnerable to being targeted by phages. This sort of turnover of the microbiome can contribute to human health and disease, but obvious connections are tenuous, given the immense variation of microbiome configurations between individuals and over the course of a lifetime. In addition, the reaction of the immune system to these abundant phages has not been evaluated to any degree.
What has been quite surprising, and seems to offer a clearer path to a direct effect on the human body, is the fact that there are many non-phage viruses that circulate in a healthy individual. These viruses are not tied to any kind of disease state – in fact, one such virus, the Torque teno virus (TTV), has recently been identified as the most abundant component of the human virome.
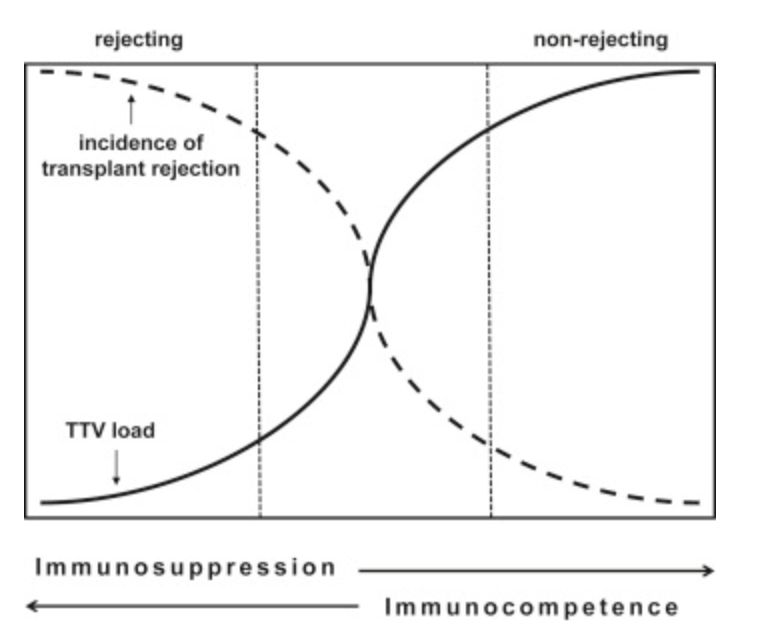
Inverse relationship between blood load of TTV and likelihood of transplant rejection.
TTVs don’t appear to cause disease, despite replicating to high copy number inside the human body. Even more interestingly – the amount of circulating TTV correlates with long-term success of organ transplantation, meaning it can be used as a readout of immune competency in advance of surgery. It also suggests that failure of organ transplantation is tied to an overactive immune system – one that is so ready to destroy foreign tissue that it would expend serious resources to attack an antigen that has no relationship to disease.
If circulating viruses that appear to have little to nothing to do with immune system aren’t enough, there’s also endogenous retroviruses. It’s well known that retroviruses, like HIV, integrate into the chromosome as part of their normal replication process. It’s also know that the human genome contains between 1-8% endogenous retroviral material – pieces of ancient viruses that have been incorporated into the genome, despite deactivation of the full viral program – cell entry, replication, cell destruction. A recent study, though far from comprehensive, suggests that viruses may play an important role in long-term genetic exchange with their hosts The study is small, but the authors suggest their findings indicate a long-term process of genetic exchange between viruses and their hosts.
The bottom line
However you slice it, researchers are starting to realize that the virome is important for health and disease, in much the same way that they realized this for the microbiome roughly 20 years ago. Sequencing techniques and approaches for studying our lifelong viral companions are just now coming into maturity, and they will seriously redefine how we will understand things like Germ theory and Koch’s postulates in the next century.
The big challenge ahead is to find ways of studying the vast swaths of new information in way that is most informative. Given the vast changes to our microscopic inhabitants over the course of lifetime, it may be that the best approach will be to leverage the era of personalized medicine in order to produce robust conclusions about how, exactly, a change in the virome can lead to a disease in a specific individual – rather in the population as a whole.
Early discoveries are tantalizing, and suggest ample correlations between changes in the virome and the onset of disease – increased blood prophages in cardiovascular disease, global changes in microbiota associated with ageing, viral drivers of Crohn’s disease, cancer, hypertension.
It is easy to imagine, at sail on the ocean of big data, that correlation is sufficient to begin moving in the direction of treatment, of producing technology, of leveraging our understanding to produce something that can be used to manipulate human health. Reading some of these papers, it seems like there are those who believe mapping the predator-prey relationships between viruses and humans will uncover the very roots of disease, allowing humans to live outside the constant shadow of death.
But I would remind those who would head in that direction of the sad state of antimicrobial research today – a lack of antibiotics, a shuttering of research programs, forecasts of superbugs death tolls that overshadow all known killers that are around today. This has been driven by a fundamental misunderstanding about the meaning of health and disease, happily taken advantage of by an endless appetite for a pill that can make you well without all the pain and struggle of behavioral changes.
Hopefully, in this next era, it will be possible to move forward with our understanding while maintaining the realization that animal models appear to overestimate the contribution of the microbiome to disease – something that could be related to the fact that something like 90% of cancer studies don’t translate between animal models and clinical trials.
I prefer to listen to the voices that remind us to continue studying these complex relational networks not because we expect to find a stable correlation between the virome sequence and disease – but because we may be surprised, and shocked that reality does not neatly dovetail with what we imagined we’ll find.